GSK announces Cervarix™ approved in China
Date: Jul 21,2016 Read:
Share:
 More exciting events are coming,
More exciting events are coming,
scan the QR code to get them!
X
GSK announces Cervarix™ approved in China to help protect women from cervical cancer.
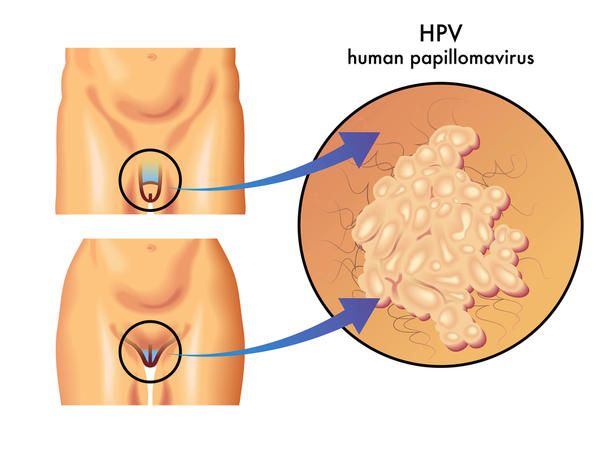
Access to human papillomavirus (HPV) vaccination could help to protect the health of millions of women in China at risk from exposure to Human Papillomavirus, the major cause of cervical cancer.
GSK China today announced that Cervarix™ (Human Papillomavirus (Types 16, 18) Vaccine, Adsorbed) ) has been approved by the China Food and Drug Administration (CFDA) as the first human papillomavirus (HPV) vaccine licensed for use in China, helping to prevent cervical cancer. Together with the cervical cancer screening programme, HPV vaccination will provide a better solution for Chinese women to fight against cervical cancer. Cervarix is registered in China for use in girls and women aged 9-25 years with a 3 dose schedule. The commercial launch of Cervarix is expected in early 2017.

A clinical trial conducted in China, involving more than 6000 subjects who received Cervarix or (Al (OH)3 control and were followed for up to 6 years, demonstrated a high efficacy and favourable benefit risk ratio in preventing certain oncogenic HPV-related cervical diseases, which is consistent with global data.1 The approval of Cervarix means that girls and young women in China will now have the chance to have access to a preventive vaccine against this deadly disease.
Hervé Gisserot, Senior Vice President and General Manager, Pharmaceuticals and Vaccines, GSK China/HongKong, said, “Increasing access to our innovative medicines and vaccines is a clear strategy for GSK China. We believe that we can work with the relevant authorities to ensure that people in China have increased access to innovative vaccines, such as Cervarix. To achieve this we are ready to explore an innovative pricing approach to support the inclusion of Cervarix into public cervical cancer immunisation programmes”.
In addition to improving access, the quality of our vaccines has always been of utmost importance. GSK China fully supports the new regulations on the administration of vaccines distribution recently issued by the Chinese government. This not only benefits the people of China, it also fulfils our commitment to be “in China, with China, for China”.
Cervical cancer is the second most common cancer in women aged between 15 to 44 years in China, with an estimated 130,000 new cases each year. Every year, China accounts for more than 28% of the world’s cervical cancer cases. Worldwide, a new case of cervical cancer is detected every minute on average, and a woman dies of cervical cancer every 2 minutes. The introduction of HPV vaccination alongside China’s cervical cancer screening programme has the potential to substantially reduce the incidence of cervical cancer and pre-cancer, and the burden of disease in China.
GSK commitment to China:
We are a science-led global healthcare company. We research, develop and manufacture a wide range of medicines, vaccines and consumer healthcare products. People are at the heart of what we do. We strive to bring high quality, high performing products to everyone who needs them. Creating innovative products and improving access to them is critical to our mission, allowing us to help build stronger, healthier communities. In China, and around the world, we are on a mission to help people do more, feel better, live longer. We are in China, with China, for China, and our commitment remains unwavering.
Some images are from network.